In adult patients with R/R B-cell precursor ALL
TECARTUS® provided deep and durable responses in ZUMA-31,2
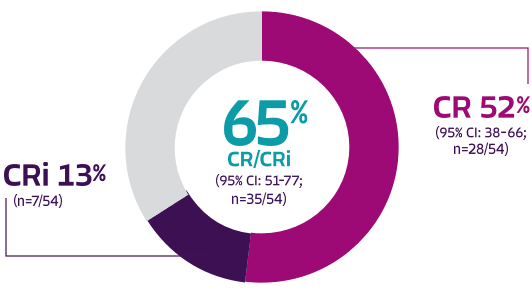
Pivotal analysis: 65% overall complete remission rate (CR + CRi) and 52% CR rate with TECARTUS at a median study follow-up of 12.3 months1
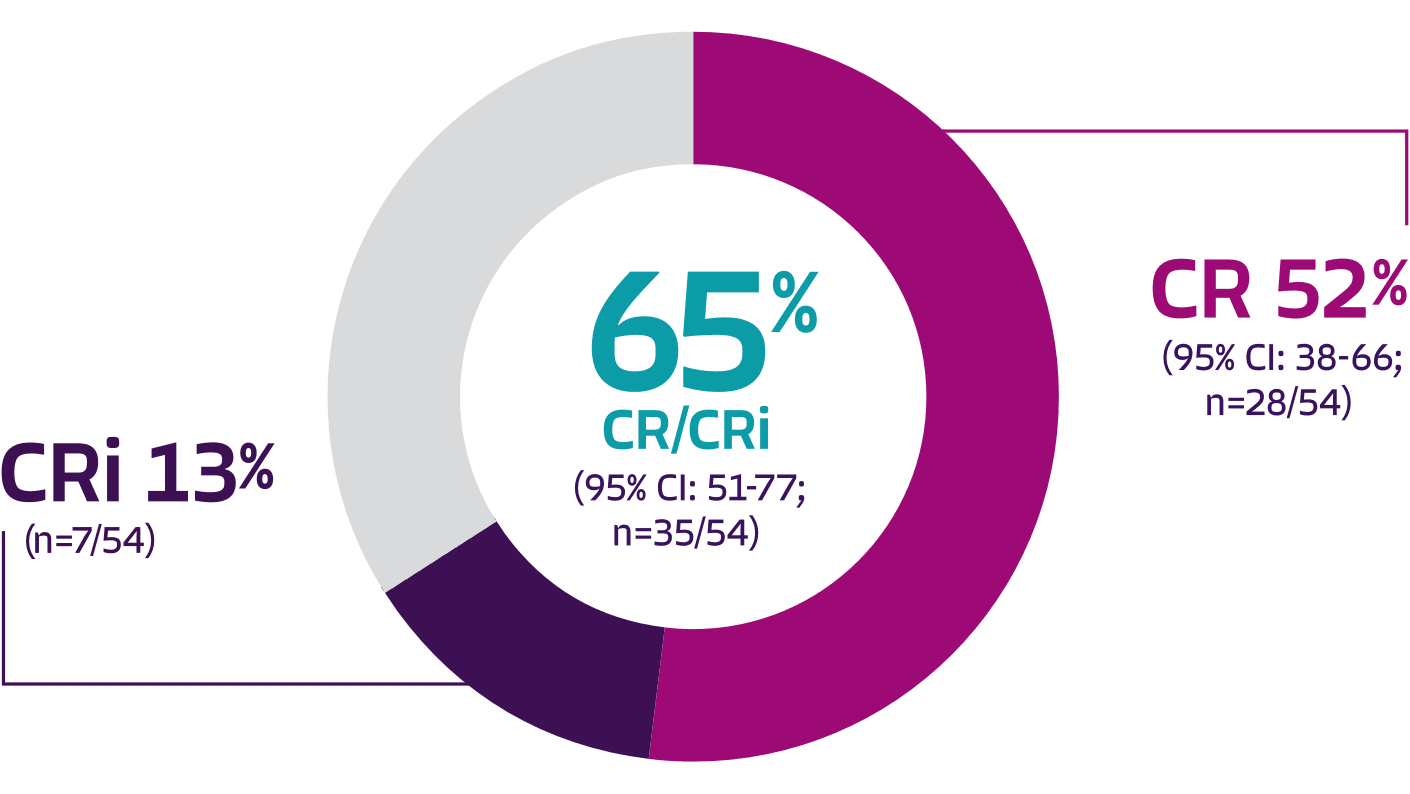
Pinch to zoom
- The ZUMA-3 primary analysis publication (n=55, efficacy analysis set) reported that TECARTUS provided high OCR rates for patients, including those who received prior treatment with blinatumomab (OCR rate=60%; n=15/25) or inotuzumab ozogamicin (OCR rate=67%; n=8/12), those who previously relapsed following allo-SCT (OCR rate=71%; n=17/24),* and those who had primary refractory disease (OCR rate=78%; n=14/18)3
Complete remission in <2 months
- Pivotal analysis: Median time to complete response was 56 days (range: 25-86 days)1
76% of patients treated with TECARTUS (42/55) were MRD-negative3
- From the primary analysis publication: MRD-negativity rate (10−4 sensitivity) was a secondary endpoint of the ZUMA-3 trial.3 The MRD assay used in this trial has not been analytically validated in accordance with the FDA guidance “Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment Guidance for Industry” issued in 2020.4 These data are not included in the TECARTUS USPI and should be carefully interpreted as further validation of the assay is needed
*Includes 1 patient who received autologous SCT.
Consistent response rates observed across efficacy analyses
Response rates in
ZUMA-3
Efficacy-evaluable population†
Pivotal analysis (n=54)
Pivotal analysis (median actual follow-up time: 12.3 months)1,3
Response rate (n) [95% Cl]
Overall complete remission rate (CR + CRi)
65% (35) [51-77]
Complete remission rate
52% (28) [38-66]
~2-year analysis (n=55)
(data cutoff July 23, 2021)2
Response rate (n) [95% Cl]
Overall complete remission rate (CR + CRi)
71% (39) [57-82]
Complete remission rate
56% (31)
ZUMA-3 overall
Pivotal analysis (n=71; all leukapheresed patients)
(median actual follow-up time: 12.3 months)1,3
Response rate (n) [95% Cl]
Overall complete remission rate (CR + CRi)
51% (36) [39-63]
Complete remission rate
41% (29) [29- 53]
~2-year analysis (n=78; pooled phase 1 and 2 treated patients)
(data cutoff July 23, 2021)2
Response rate (n) [95% Cl]
Overall complete remission rate (CR + CRi)
73% (57) [62-82]
Complete remission rate
60% (47)
Efficacy-evaluable population†
(n=54)
(n=55)
Response rate (n) [95% Cl]
Pivotal analysis
(median actual follow-up time:
12.3 months)1,3
~2-year analysis
(data cutoff July 23, 2021)2
Overall complete remission rate (CR + CRi)
Complete remission rate
65% (35) [51-77]
52% (28) [38-66]
71% (39) [57-82]
56% (31)
ZUMA-3 overall
(n=71;
all leukapheresed patients)
(n=78;
pooled phase 1 and 2 treated patients)
Response rate (n) [95% Cl]
Pivotal analysis
(median actual follow-up time:
12.3 months)1,3
~2-year analysis
(data cutoff July 23, 2021)2
Overall complete remission rate (CR + CRi)
Complete remission rate
51% (36) [39-63]
41% (29) [29-53]
73% (57) [62-82]
60% (47)
†Of the 71 patients who were enrolled (and leukapheresed), 57 patients received lymphodepleting chemotherapy, and 55 patients received TECARTUS. 54 patients were included in the efficacy-evaluable population.1
ALL=acute lymphoblastic leukemia; allo-SCT=allogeneic stem cell transplant; CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete hematologic recovery; DOR=duration of remission; FDA=Food and Drug Administration; MRD=minimal residual disease; OCR=overall complete remission; R/R=relapsed or refractory; USPI=US Prescribing Information.
References: 1. TECARTUS® (brexucabtagene autoleucel). Prescribing information. Kite Pharma, Inc; 2025. 2. Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol. 2022;15(1):170. 3. Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. 4. Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment Guidance for Industry. Oncology Center of Excellence in cooperation with the Center for Drug Evaluation and Research and the Center for Biologics Evaluation and Research, Food and Drug Administration, US Dept of Health and Human Services; January 2020.
~2-year analysis (median follow-up: 26.8 months)*: TECARTUS provided a median DOR of 14.6 months2
- DOR was a secondary endpoint of the ZUMA-3 phase 2, single-arm, open-label study1,3
- DOR data are descriptive and should be carefully interpreted in light of the single-arm study design. DOR data from the ~2-year analysis were by investigator assessment and are not included in the USPI
- ~2-year follow-up analysis was conducted in the 55 efficacy-evaluable patients treated with TECARTUS in the ZUMA-3 study2
Adapted from Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study, J Hematol Oncol, 2022;15(1):170, © 2022 The Author(s), https://creativecommons.org/licenses/by/4.0. The copyright owner disclaims all representations and warranties regarding the licensed material.
- mDOR of 8.7 months in patients with a CRi (n=8 responders; 95% CI: 1.0-NE)2
Pinch to zoom
Pivotal analysis1
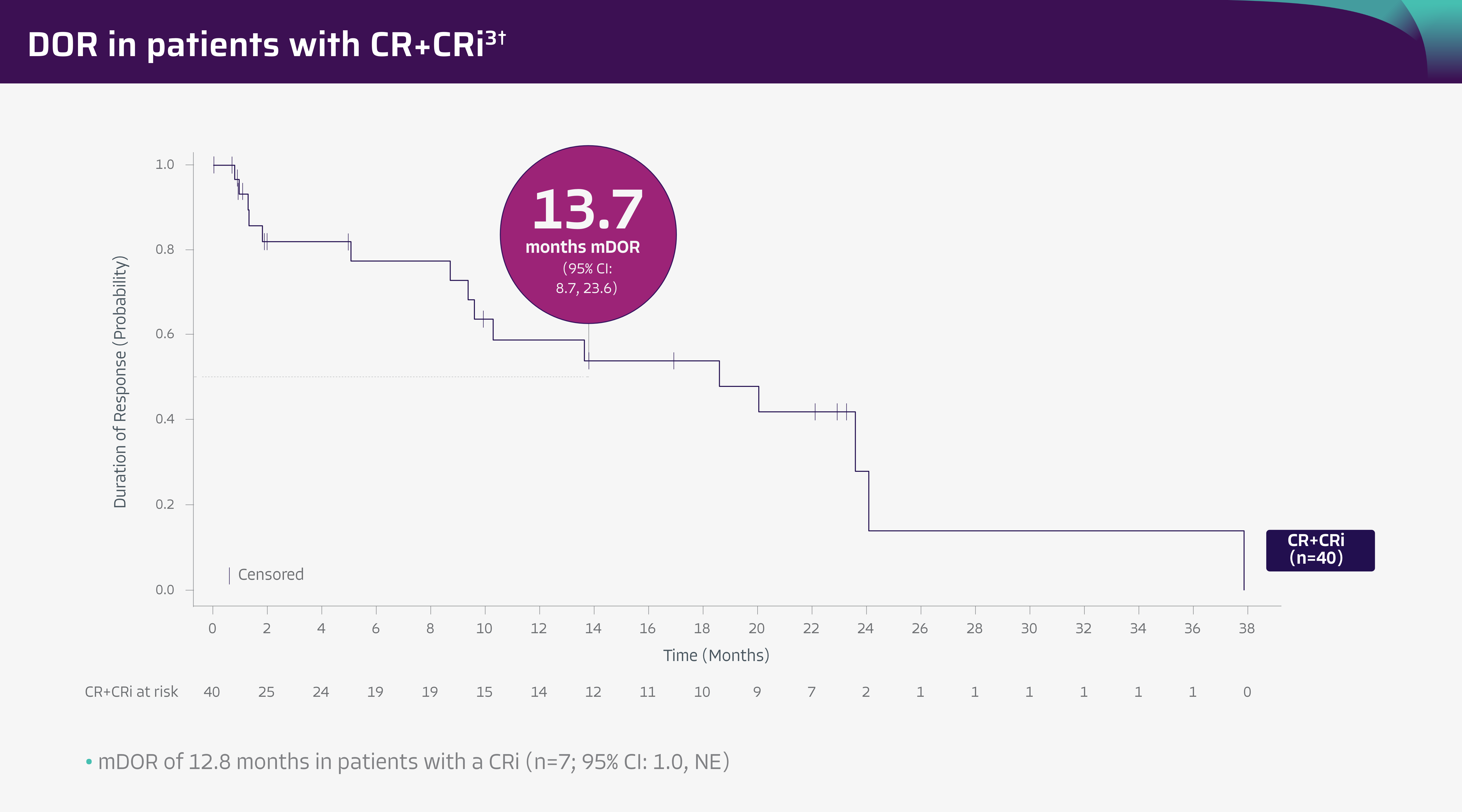
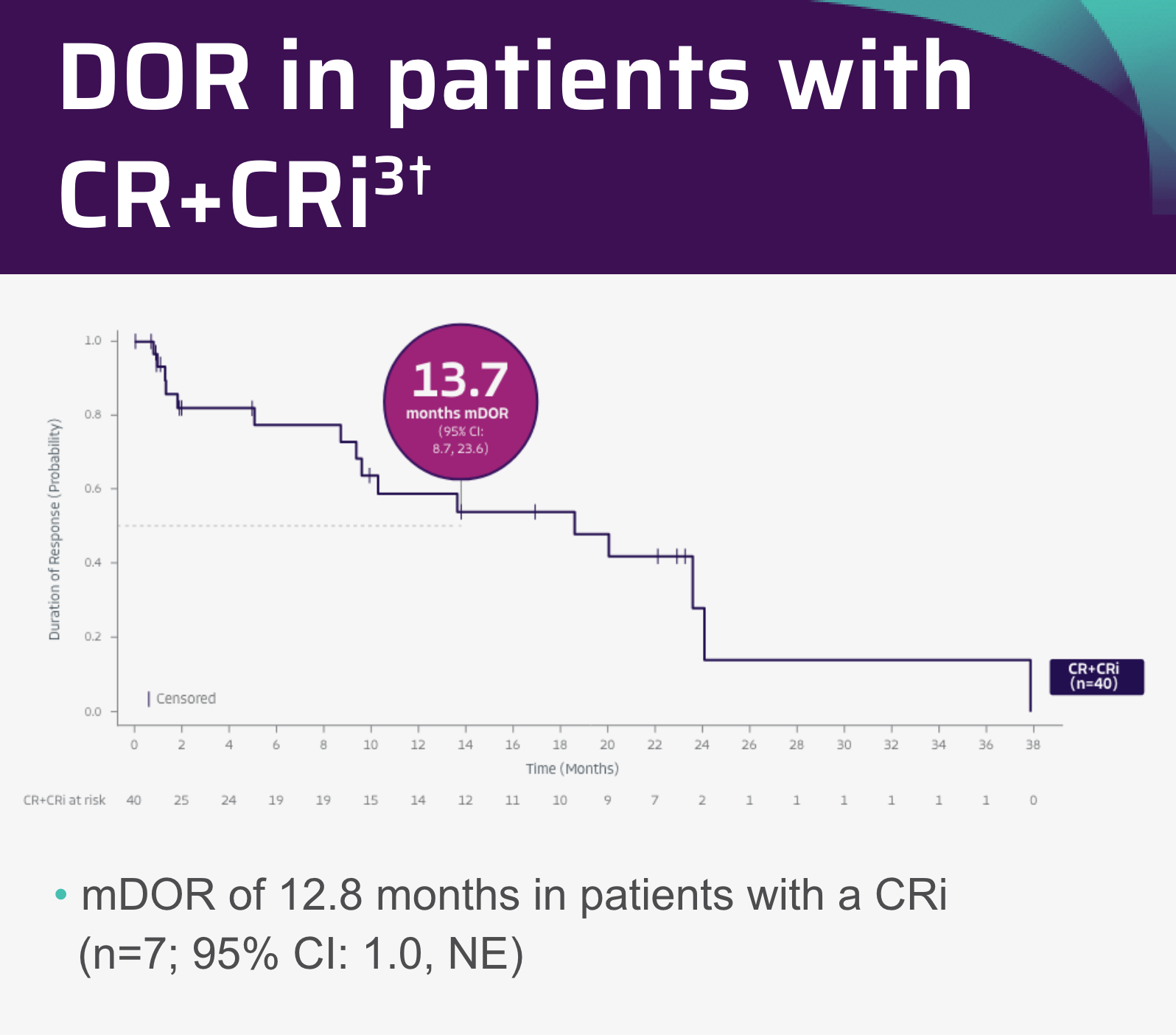
- In the pivotal analysis (median study follow-up: 12.3 months), mDOR was 13.6 months in both efficacy-evaluable patients (n=54; 95% CI: 9.4-NE) and in all leukapheresed patients (n=71; 95% CI: 8.7-NE)
- Median DOR was not reached in patients with a CR (95% CI: 9.6-NE) in efficacy-evaluable patients (n=54) and was 13.6 months (95% CI: 9.4-NE) in all leukapheresed patients (n=71)
- Median DOR was 8.7 months in patients with a CRi (95% CI: 1.0-NE) in both efficacy-evaluable patients (n=54) and all leukapheresed patients (n=71)
*Potential follow-up time.2
~2-year analysis*: patients achieved 18.6 months mDOR without censoring for subsequent allo-SCT2
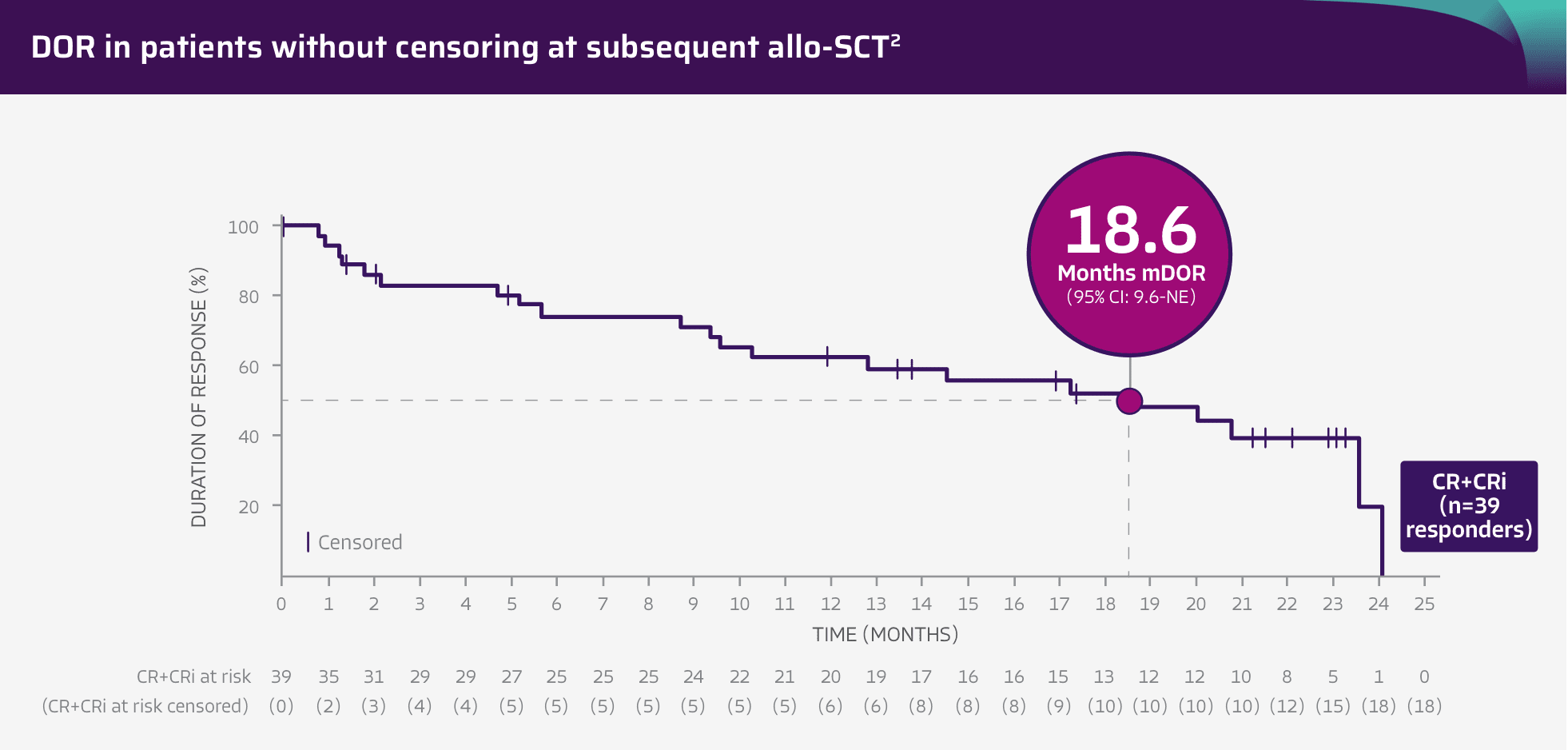
Pinch to zoom
Adapted from Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study, J Hematol Oncol, 2022;15(1):170, © 2022 The Author(s), https://creativecommons.org/licenses/by/4.0. The copyright owner disclaims all representations and warranties regarding the licensed material.
- mDOR of 20.8 months in patients with a CR (n=31; 95% CI: 14.6-NE) and mDOR of 5.7 months in patients with a CRi (n=8; 95% CI: 1.0-12.8)2
- DOR was a secondary endpoint of the ZUMA-3 phase 2, single-arm, open-label study1,3
- DOR data are descriptive and should be carefully interpreted in light of the single-arm study design
- The study was not designed to assess efficacy of subsequent allo-SCT or to make comparisons between TECARTUS and allo-SCT, as no head-to-head trial has been conducted
ALL=acute lymphoblastic leukemia; allo-SCT=allogeneic stem cell transplant; CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete hematologic recovery; DOR=duration of remission; mDOR=median duration of remission; NE=not estimable; OCR=overall complete remission; R/R=relapsed or refractory; USPI=US Prescribing Information.
References: 1. TECARTUS® (brexucabtagene autoleucel). Prescribing information. Kite Pharma, Inc; 2025. 2. Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol. 2022;15(1):170. 3. Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502.