IMPORTANT SAFETY INFORMATION
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, AND SECONDARY HEMATOLOGICAL MALIGNANCIES
- Cytokine Release Syndrome (CRS), including life-threatening reactions, occurred in patients receiving TECARTUS. Do not administer TECARTUS to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids.
- Neurologic toxicities, including life-threatening reactions, occurred in patients receiving TECARTUS, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with TECARTUS. Provide supportive care and/or corticosteroids as needed.
- T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies.
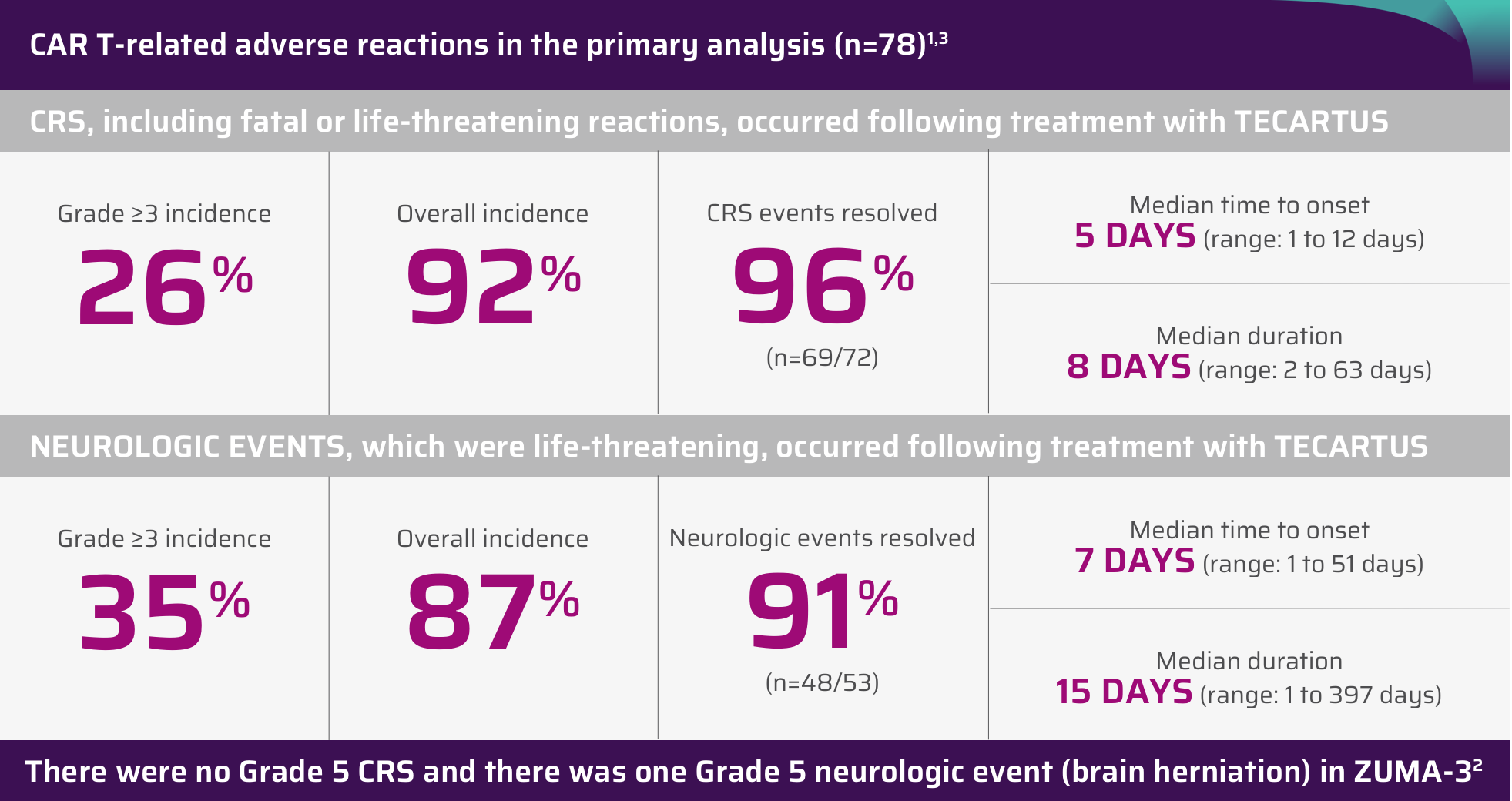
CYTOKINE RELEASE SYNDROME (CRS)CRS, including fatal or life-threatening reactions, occurred following treatment with TECARTUS. CRS occurred in 92% (72/78) of patients with ALL, including ≥ Grade 3 CRS in 26% of patients. Three patients with ALL had ongoing CRS events at the time of death. The median time to onset of CRS was 5 days (range: 1 to 12 days) and the median duration of CRS was 8 days (range: 2 to 63 days). The incidence of CRS (first occurrence) within the first 7 days after TECARTUS infusion was 90% (70/78) in patients with ALL.
Among patients with CRS, key manifestations (>10%) included fever (93%), hypotension (62%), tachycardia (59%), chills (32%), hypoxia (31%), headache (21%), fatigue (20%), and nausea (13%). Serious events associated with CRS included hypotension, fever, hypoxia, tachycardia, and dyspnea. Serious events associated with CRS in all combined patients (≥2%) included hypotension, fever, hypoxia, tachycardia, and dyspnea.
Confirm that a minimum of two doses of tocilizumab are available for each patient prior to infusion of TECARTUS. Monitor patients daily for at least 7 days following infusion for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for 2 weeks after infusion. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated.
NEUROLOGIC TOXICITIESNeurologic toxicities including immune effector cell-associated neurotoxicity syndrome (ICANS) that were fatal or life-threatening, occurred following treatment with TECARTUS. Neurologic events occurred in 87% (68/78) of patients with ALL, including ≥ Grade 3 in 35% of patients. The median time to onset for neurologic events was 7 days (range: 1 to 51 days) with a median duration of 15 days (range: 1 to 397 days) in patients with ALL. The onset of neurologic events can be concurrent with CRS following resolution of CRS or in the absence of CRS. Ninety-one percent of all treated patients experienced the first CRS or neurological event within the first seven days after TECARTUS infusion.
The most common neurologic events (>10%) included encephalopathy (57%), headache (37%), tremor (34%), confusional state (26%), aphasia (23%), delirium (17%), dizziness (15%), anxiety (14%), and agitation (12%). Serious events associated with neurologic toxicities in MCL and ALL (≥ 2%) including encephalopathy, aphasia, confusional state, and seizures occurred after treatment with TECARTUS.
Monitor patients daily for at least 7 days following infusion for signs and symptoms of neurologic toxicity/ICANS. Monitor patients for signs or symptoms of neurologic toxicities for 2 weeks after infusion and treat promptly. Advise patients to avoid driving for at least 2 weeks following infusion.
HEMOPHAGOCYTIC LYMPHOHISTIOCYTOSIS/MACROPHAGE ACTIVATION SYNDROME (HLH/MAS)HLH/MAS, including life-threatening reactions, occurred following treatment with TECARTUS. HLH/MAS occurred in 4% (3/78) of patients with ALL. Two patients experienced Grade 3 events and 1 patient experienced a Grade 4 event. The median time to onset for HLH/MAS was 8 days (range: 6 to 9 days) with a median duration of 5 days (range: 2 to 8 days). All 3 patients with HLH/MAS had concurrent CRS symptoms and neurologic events after TECARTUS infusion. Treatment of HLH/MAS should be administered per institutional standards.
HYPERSENSITIVITY REACTIONSSerious hypersensitivity reactions, including anaphylaxis, may occur due to dimethyl sulfoxide (DMSO) or residual gentamicin in TECARTUS.
SEVERE INFECTIONSSevere or life-threatening infections occurred in patients after TECARTUS infusion. Infections (all grades) occurred in 44% (34/78) of patients with ALL. Grade 3 or higher infections, including bacterial, viral, and fungal infections, occurred in 30% of all combined patients. TECARTUS should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after infusion and treat appropriately. Administer prophylactic antimicrobials according to local guidelines.
Febrile neutropenia was observed in 35% of patients with ALL after TECARTUS infusion and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
In immunosuppressed patients, life-threatening and fatal opportunistic infections have been reported. The possibility of rare infectious etiologies (e.g., fungal and viral infections such as HHV-6 and progressive multifocal leukoencephalopathy) should be considered in patients with neurologic events and appropriate diagnostic evaluations should be performed.
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with drugs directed against B cells. Perform screening for HBV, hepatitis C virus (HCV), and human immunodeficiency virus (HIV) in accordance with clinical guidelines before collection of cells for manufacturing.
PROLONGED CYTOPENIASPatients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and TECARTUS infusion. In patients with ALL who were responders to TECARTUS treatment, Grade 3 or higher cytopenias not resolved by Day 30 following TECARTUS infusion occurred in 20% (7/35) of patients and included neutropenia (12%) and thrombocytopenia (12%); Grade 3 or higher cytopenias not resolved by Day 60 following TECARTUS infusion occurred in 11% (4/35) of patients and included neutropenia (9%) and thrombocytopenia (6%). Monitor blood counts after TECARTUS infusion.
HYPOGAMMAGLOBULINEMIAB-cell aplasia and hypogammaglobulinemia can occur in patients receiving treatment with TECARTUS. Hypogammaglobulinemia was reported in 9% (7/78) of patients with ALL. Monitor immunoglobulin levels after treatment with TECARTUS and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement.
The safety of immunization with live viral vaccines during or following TECARTUS treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during treatment, and until immune recovery following treatment with TECARTUS.
SECONDARY MALIGNANCIESPatients treated with TECARTUS may develop secondary malignancies. T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies. Mature T cell malignancies, including CAR-positive tumors, may present as soon as weeks following infusions, and may include fatal outcomes.
Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
ADVERSE REACTIONSThe most common non-laboratory adverse reactions (≥ 20%) were fever, CRS, hypotension, encephalopathy, tachycardia, nausea, chills, headache, fatigue, febrile neutropenia, diarrhea, musculoskeletal pain, hypoxia, rash, edema, tremor, infection with pathogen unspecified, constipation, decreased appetite, and vomiting.
Please see full Prescribing Information, including BOXED WARNING and Medication Guide.