Acute lymphoblastic leukemia (ALL) is an aggressive, rare,
heterogeneous, rapidly progressing disease1-3
~50%
of ALL cases occur
in adults ≥18 years old4
~3000 adult patients are
diagnosed each year4,5
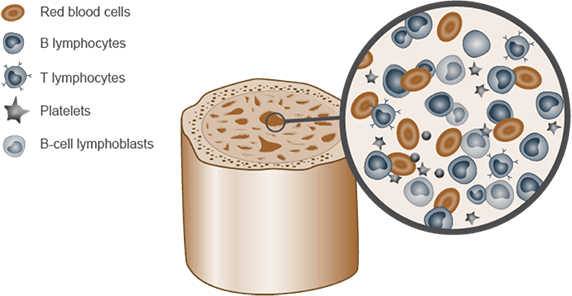
ALL develops from malignant cells within lymphocytes in the bone marrow3
- Abnormal levels of T- or B-cell lymphoblasts accumulate in the bone marrow3
- Diagnosis of ALL generally requires ≥20% of bone marrow to contain lymphoblasts1
Pinch to zoom
Adapted from National Cancer Institute. Adult acute lymphoblastic
leukemia treatment (PDQ®)-health professional version3
While ALL originates in the bone marrow, it can migrate to the lymph nodes, liver, spleen, and central nervous system3
Patient demographics
- Median age among adults is 37 years2
- More common in Hispanics and Whites5
- ~30% more common in males5
Most patients present with cytopenia-related symptoms; additional signs and symptoms of ALL include1,3:
- Weight loss
- Fever
- Night sweats
- Bone or joint pain
- Lymphadenopathy
- Splenomegaly
- Hepatomegaly
- Difficulty breathing
Evaluations used for ALL diagnosis and risk assessment1
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend evaluation and treatment at specialized treatment centers with expertise in ALL management1
Diagnostic testing1
- Physical and labs
- CBC, DIC panel, TLS panel, screen for infections, testicular exam, etc
- Morphologic assessment of bone marrow
- Hematopathology review to determine blast ratio
- Immunophenotyping
- Flow cytometry to identify ALL subtype
- Cytogenetic analysis
- Karyotyping to determine presence of high-risk features
- Lumbar puncture
- To determine presence of extramedullary disease
Pathological workup for risk stratification1
- Molecular characterization
- FISH testing for genetic abnormalities
- RT-PCR testing for BCR-ABL1 mutation
- Next generation sequencing for gene fusions and pathogenic mutations
Minimal residual
disease (MRD) testing and monitoring1
- MRD is the presence of leukemic cells below the detection threshold of ≥10-4 via conventional morphologic methods
- Assess using bone marrow aspirate via flow cytometry, PCR, or next-generation sequencing (NGS)
- An essential component of patient evaluation over the course of sequential therapy
- Recommended by the NCCN Guidelines in patients with R/R disease after initial therapy, and at additional timepoints (dependent upon treatment regimen)
Classification of adult ALL is based on immunophenotype
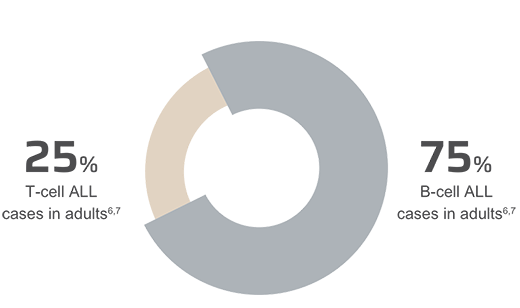
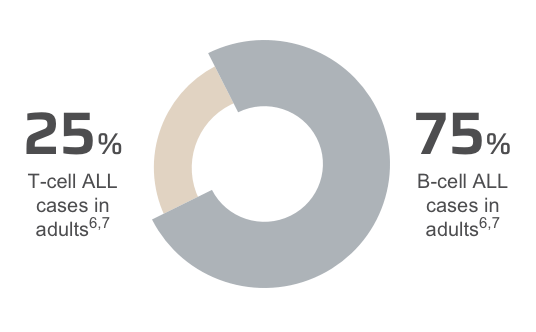
Pinch to zoom
B-cells express the cell surface
antigen CD19 throughout their
maturation process6,8
There are 4 key risk factors that affect the prognosis and management of B-cell precursor ALL in adults9-11
Age9
Outcomes in adults with ALL worsen with increasing age
Cytogenetics1
Expression of certain cytogenetic abnormalities such as IKZF1, BCR-ABL-1-like, and KMT2A are associated with worse outcomes
Minimal Residual Disease (MRD) status10
MRD positivity is associated with poor outcomes, including low survival rates and high risk of relapse
Time to relapse11
Patients with early relapse have worse outcomes than patients with late relapse
Presence of these risk factors are often associated with poor survival and higher risk of relapse1,9-12
ALL=acute lymphoblastic leukemia; BCR-ABL=breakpoint cluster region Abelson; CBC=complete blood count; CNS=central nervous system; DIC=disseminated intravascular coagulation; FISH=fluorescence in situ hybridization; IKZF1=Ikaros gene; KMT2A=lysine methyltransferase 2A; MLL=mixed lineage leukemia; MRD=minimal residual disease; NCCN=National Comprehensive Cancer Network; PCR=polymerase chain reaction; R/R=relapsed or refractory; RT PCR=reverse transcriptase-polymerase chain reaction; TLS=tumor lysis syndrome.
References: 1. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Lymphoblastic Leukemia V.2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed July 15, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. 2. Pulte D, Jansen L, Gondos A, et al. Survival of adults with acute lymphoblastic leukemia in Germany and the United States. PLoS One. 2014;9(1):e85554. doi:10.1371/journal.pone.0085554 3. National Cancer Institute. Adult acute lymphoblastic leukemia treatment (PDQ®)-health professional version. https://www.cancer.gov/types/leukemia/hp/adult-all-treatment-pdq. Updated June 30, 2023. Accessed August 4, 2023. 4. SEER*Explorer. Incidence and mortality comparison. National Cancer Institute. https://seer.cancer.gov/explorer/. Updated June 8, 2023. Accessed August 4, 2023. 5. National Cancer Institute. Cancer stat facts: leukemia — acute lymphocytic leukemia (ALL). https://seer.cancer.gov/statfacts/html/alyl.html. Accessed August 4, 2023. 6. Bassan R, Gatta G, Tondini C, Willemze R. Adult acute lymphoblastic leukaemia. Crit Rev Oncol Hematol. 2004;50(3):223-261. 7. Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350(15):1535-1548. 8. Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1(1):36. Published November 29, 2012. doi:10.1186/2162-3619-1-36 9. SEER*Explorer. Survival. National Cancer Institute. https://seer.cancer.gov/explorer/. Updated June 8, 2023. Accessed August 4, 2023. 10. Brüggemann M, Kotrova M. Minimal residual disease in adult ALL: technical aspects and implications for correct clinical interpretation. Blood Adv. 2017;1(25):2456-2466.11. Ganzel C, Wang XV, Rowe JM, et al. At three years, patients with acute lymphoblastic leukaemia are still at risk for relapse. Results of the international MRC UKALLXII/ECOG E2993 trial. Br J Haematol. 2020;191(1):37-43. 12. Saygin C, Papadantonakis N, Cassaday RD, et al. Prognostic impact of incomplete hematologic count recovery and minimal residual disease on outcome in adult acute lymphoblastic leukemia at the time of second complete response. Leuk Lymphoma. 2018;59(2):363-371.