MCL is a rare and aggressive B-cell lymphoma1
Nearly all patients will either relapse on or be refractory to 1L therapy, and outcomes worsen with each subsequent line of therapy2
At diagnosis, outcomes are worse in patients with high-risk characteristics3,4
| Risk factor | mOS in patients with high-risk characteristics vs patients without |
|---|---|
| TP53 mutations* | ~2× shorter (42 months vs 80 months, respectively; P<0.0001) |
| Blastoid morphology* | ~3× shorter (26 months vs 75 months, respectively; P<0.0001) |
| Ki-67 index ≥30%† | 3.4 years with Ki-67 ≥30% vs not reached with Ki-67 <30% |
*Data from a real-world study of 4049 patients diagnosed with MCL from 2011 through 2021 and treated with chemotherapy, BTKis, proteasome inhibitors, IMiDs, and Bcl2 inhibitor alone or in combination with other therapies in predominantly community-based oncology practices in the United States. Of 4049 patients, 278 (7%) did not have treatment documented at last follow-up.3
†Results of a retrospective analysis of data from 508 patients with MCL registered in either the MCL Elderly trial (aged ≥60 years) or MCL Younger trial (aged <66 years) with Ki-67 index available (median follow-up of 5.7 years). Patients in MCL Younger were treated with HDT + ASCT.4
Limitations
Analyses subject to limitations inherent in retrospective studies (eg, missing data, selection bias). In the real-world study in 4049 patients (Narkhede et al.), nearly half of the patients had data missing for at least 1 of the known prognostic factors (eg, Ki-67, TP53 mutations status), which may have led to a selection bias. Mortality data are often difficult to accurately collect in a real-world setting; for this study, data were based on a composite of EHR-derived data, obituary data, and the Social Security Death Index. Due to the retrospective nature of these studies, histopathologic characteristics were not available for all patients. However, sensitivity analyses were performed and multiple imputation of missing histopathologic variables consistently confirmed the results obtained with the cases and the complete data.
While evolving therapies‡ have improved outcomes in R/R MCL, relapse is almost universal1,2
of patients
treated with BTKi progress
within 12-18 months5,6§
‡National Comprehensive Cancer Network® (NCCN®)–suggested regimens for 2L R/R MCL include: covalent BTKis (acalabrutinib, zanubrutinib, ibrutinib), lenalidomide + rituximab, bendamustine + rituximab, R-BAC 500, bortezomib +/- rituximab, GemOx + rituximab, R-DHAP, ibrutinib + venetoclax, venetoclax +/- rituximab, and ibrutinib + rituximab.1
§Data from 2 studies evaluating BTKi therapy in R/R MCL: (1) an open-label, multicenter, phase 3 clinical trial in 280 patients with R/R MCL, who had received ≥1 rituximab-containing treatments, randomized to receive ibrutinib (n=139) or temsirolimus (n=141). The primary efficacy endpoint was PFS. (2) An open-label, single-arm, multicenter, phase 2 clinical trial in 124 patients with R/R MCL who received acalabrutinib. The primary endpoint was overall response (assessed by Lugano classification). PFS was a secondary endpoint.5,6
Shortening of OS and PFS with each relapse observed in a retrospective study2¶
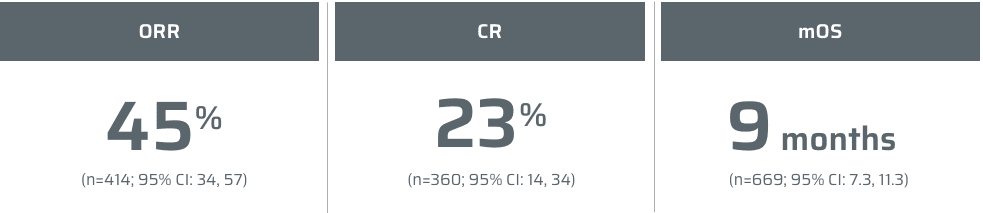
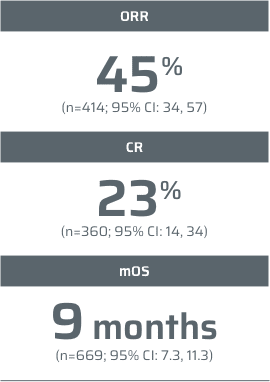
¶Data from a retrospective study of 404 consecutive patients with MCL who were managed between 2000 and 2014 at Memorial Sloan Kettering Cancer Center.2
Limitations
Analysis subject to limitations inherent in a retrospective study (eg, selection bias). Additionally, ibrutinib was only FDA approved in 2013 and was not available for all patients at the time of relapse outside of clinical trials. Therefore, there was a relatively small proportion of patients who received ibrutinib-based regimens in this analysis.
There remains a critical need to plan for 2L relapse with a treatment
that can improve responses for patients with R/R MCL2
1L=first-line; 2L=second-line; 3L=third-line; ASCT=autologous stem cell transplantation; Bcl2=B-cell lymphoma 2; BTKi=Bruton’s tyrosine kinase inhibitor; CAR=chimeric antigen receptor; CI=confidence interval; CR=complete response; EHR=electronic health record; FDA=Food and Drug Administration; GemOX=gemcitabine hydrochloride and oxaliplatin; HDT=high-dose chemotherapy; IMiDs=immunomodulatory drugs; LoT=line of therapy; MCL=mantle cell lymphoma; mOS=median overall survival; mPFS=median progression-free survival; NCCN=National Comprehensive Cancer Network; ORR=objective response rate; OS=overall survival; PFS=progression-free survival; R-BAC=rituximab, bendamustine, and cytarabine; R-DHAP=rituximab, dexamethasone, cytarabine, and cisplatin; R/R=relapsed or refractory.
References: 1. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas. V.2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed February 12, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. 2. Kumar A, Sha F, Toure A, et al. Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: progressive shortening in response duration and survival after each relapse. Blood Cancer J. 2019;9(50):1-10. 3. Narkhede M, Goyal G, Shea L, Mehta A, Giri S. Evaluating real-world treatment patterns and outcomes of mantle cell lymphoma. Blood Adv. 2022;6(14):4122-4131. 4. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34(12):1386-1394. 5. Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659-667. 6. Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387:770-778.