In adult patients with relapsed or refractory (R/R) mantle cell lymphoma
TECARTUS® provided deep and durable responses1
Primary analysis: In ZUMA-2, nearly all patients achieved a response (n=52/60) with TECARTUS at a median study follow-up of 12.3 months1,2
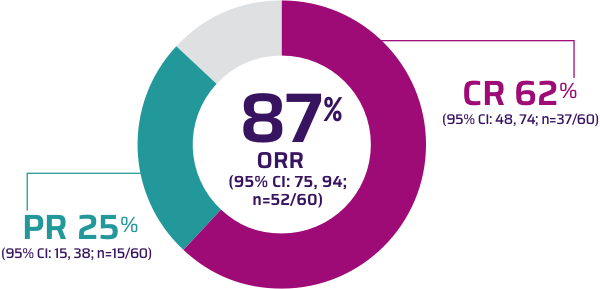
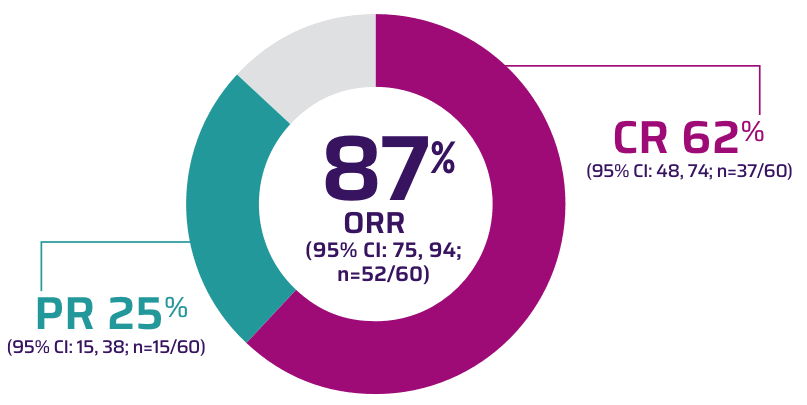

Infusion to response in 1 month1*
- Median time from infusion to complete response: 3.0 months (range: 0.9, 9.3)2
~3-year follow-up: Consistent response rate was observed in ZUMA-23†



Study limitations
These results represent a separate, preplanned, post hoc analysis of the ZUMA-2 study. Data from the ~3-year analysis are descriptive and the study was not powered or adjusted for multiplicity to assess efficacy in this follow-up study. These data are not included in the Prescribing Information for TECARTUS and should be carefully interpreted.
*Median: 28 days (range: 24, 92).1
†Median follow-up of 35.6 months (range: 25.9, 56.3).3
CI=confidence interval; CR=complete response; DOR=duration of response; MCL=mantle cell lymphoma; ORR=objective response rate; PR=partial response; R/R=relapsed or refractory.
References: 1. TECARTUS® (brexucabtagene autoleucel). Prescribing information. Kite Pharma, Inc; 2025. 2. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle cell lymphoma. N Engl J Med. 2020;382(14):1331-1342. 3. Wang M, Munoz J, Goy A, et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol. 2023;41(3):555-567.
TECARTUS provided 28.2 months mDOR, at ~3 years median study follow-up2*†
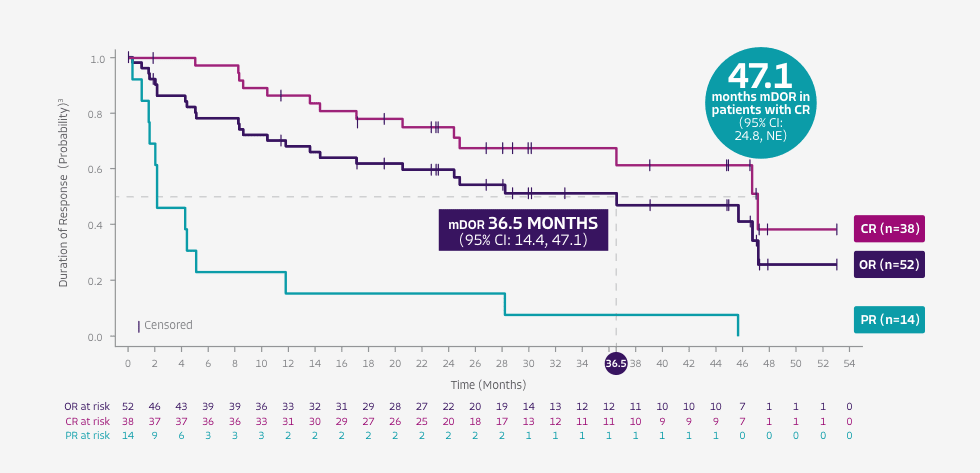
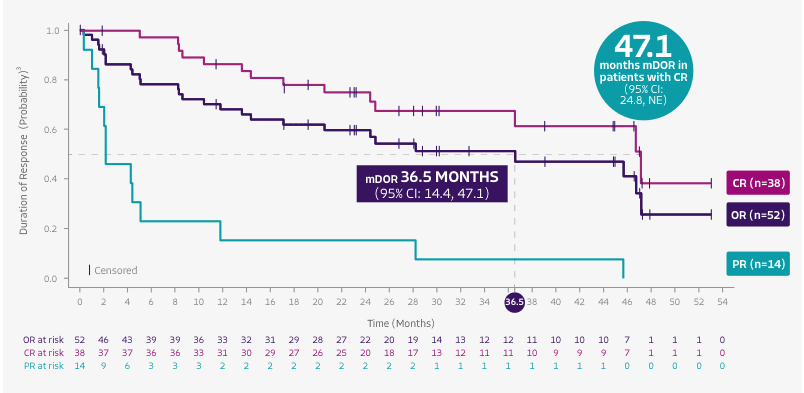
Tick marks represent censored patients. Patients not meeting criteria for progression or death by the analysis cutoff date were censored at their last evaluable disease assessment date, or were censored due to being lost to follow-up, withdrawal of consent, or initiating subsequent therapy (including SCT).3

- DOR was a secondary endpoint of the ZUMA-2 phase 2, single-arm, open-label study4
- DOR data from the ~3-year analysis are not included in the USPI. DOR data are descriptive and should be carefully interpreted
- ~3-year median study follow-up analysis was conducted in all 68 patients treated with TECARTUS in the ZUMA-2 study2
- In the primary analysis, mDOR was not reached (95% CI: 8.6, NE) at a median study follow-up of 12.3 months; number of responders=52/601,4
Study limitations
These results represent a separate, preplanned, post hoc analysis of the ZUMA-2 study. Data from the ~3-year analysis are descriptive and the study was not powered or adjusted for multiplicity to assess efficacy in this follow-up study. These data are not included in the Prescribing Information for TECARTUS and should be carefully interpreted.
*Kaplan-Meier estimate based on the 62 patients with a response. Median duration of study follow-up was 35.6 months (range, 25.9, 56.3).2
†DOR was measured from the start of first objective response to the date of progression or death.1
CI=confidence interval; CR=complete response; DOR=duration of response; mDoCR=median duration of complete response; mDOR=median duration of response; NE=not estimable; OR=overall response; ORR=objective response rate; PR=partial response; SCT=stem cell transplant; USPI=US Prescribing Information.
References: 1. TECARTUS® (brexucabtagene autoleucel). Prescribing information. Kite Pharma, Inc; 2025. 2. Wang M, Munoz J, Goy A, et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol. 2023;41(3):555-567. 3. Wang M, Munoz J, Goy A, et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol. 2023;41(3):555-567 (suppl). doi:10.1200/JCO.21.0237 4. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle cell lymphoma. N Engl J Med. 2020;382(14):1331-1342.